Femarelle® official launch in Singapore

24 October 2024, official launch of the Femarelle® line – Rejuvenate, Recharge, and Unstoppable – with Professor Andrea Genazzani and Dr June Tan Sheren. The Femarelle® line uses the proprietary ingredient DT56a, which works in the body in a selective manner, supporting the management of symptoms associated with hormonal changes across the different stages of a woman’s life, from peri-menopause to post-menopause. Femarelle® provides a unique non-hormonal botanical solution, that supports healthy female hormonal and mood balance, improves energy levels, and overall quality of life.
Dicoflor elle – Probiotic Support for Women’s Intimate Health

10 March 2025 – We are proud to announce the launch of Dicoflor elle, a targeted probiotic formulation specially developed to support and maintain women’s vaginal and urinary tract health. Dicoflor elle contains Chr. Hansen’s clinically proven probiotic strains – Lactobacillus rhamnosus GR-1® and Lactobacillus reuteri RC-14® – the most extensively studied strains for promoting a healthy vaginal microbiome and urogenital balance. Dicoflor elle is now available at selected doctors’ clinics.
UITC Launches Anscare™ LeniScar Silicone Stick

25 February 2025, UITC introduced Anscare™ LeniScar Silicone Stick, an advanced silicone balm formula with Vitamin C and E. LeniScar an easy-to-use topical balm intended to diminish scars and keloids characterised by a raised or discoloured appearance. LeniScar creates an invisible, transparent thin film which helps to maintain the moisture balance of skin and provides an optimal barrier for the scar to heal. LeniScar is available at selected doctors’ clinics.
Fight Against the First Sign of Cold & Cough with VITAHERB® Syrups

3 Feb 2025, Vitaherb® range of syrups are produced by GMP certified manufacturer, Winwa Medical, and contains only standardized, patented ingredients sources from renown suppliers from Europe. UITC and Winwa Medical are dedicated to providing patients and consumers with the very best of natural herbal medications. Natural and effective, Vitaherb® syrups are now available at leading pharmacies, and selected doctors’ clinics.
Introducing Lysoe® 90mg, Now in a Convenient Pack Size of 20’s

2 Jan 2025, we are excited to announce the launch of Lysoe® 90mg, a trusted natural enzyme now available in a convenient 20-tablets pack. Lysozyme is a naturally occurring enzyme that is widely used to soothe the throat. This natural, non-drowsy, sugar-free convenient pack is now available at leading pharmacies.
Bonjesta® official launch in Singapore

29 July 2024, official launch of Bonjesta®, with Dr Jason Lim, Professor Yariv Yogev, and A/Prof Tan Thiam Chye. Bonjesta® multilayer, extended-release tablets are designed to provide immediate and continuous relief of morning sickness symptoms throughout the day and night. The simple schedule of administration reduces pill burden and improves patient compliance.
UITC launches Anscare™ Scar Reduction Silicone Gel C+
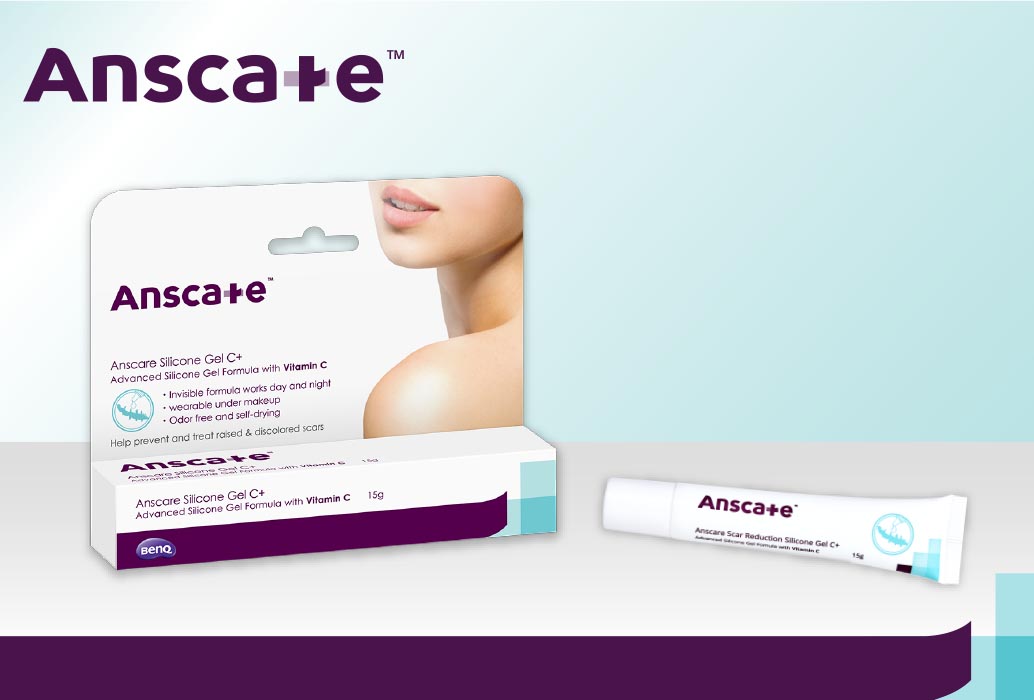
10 January 2024, UITC launches BenQ Anscare Scar Reduction Silicone Gel C+, an advanced silicone gel formula with Vitamin C that softens and flattens both existing and new scars, like keloids and hypertrophic scars that result from surgery injury, C-section, burns, cuts, scrapes, acnes, insect bites. Available at doctors’ clinics.
UITC expands Bioflor® range with Bioflor® capsules

4 November 2023, in addition to Bioflor® sachets, UITC has also launched Bioflor® capsules, which is a more convenient dosage form for adults. Bioflor Saccharomyces boulardii CNCM I-745® is a unique yeast based probiotic drug that is paired together with antibiotics, used for the treatment and prevention of antibiotic-associated diarrhoea.
Bonjesta® regulatory approval by HSA

18 September 2023, UITC is pleased to announce regulatory approval has been obtained for Bonjesta®. Bonjesta® is a faster acting, longer lasting, optimized reformulation of Diclectin®, indicated for the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative management.
K-CAB® official launch in Singapore

31 August 2023, official launch of K-CAB®, with Professor Hwoon-Yong Jung, Professor Chandra Prakash Gyawali, and Adjunct Associate Professor David Ong. K-CAB® is a novel drug in the new P-CAB class, used for the treatment of acid-related gastrointestinal disorders. It is characterized by its rapid onset of action within 30 minutes and sustained nocturnal gastric acid control.